History
Patients with alcoholic hepatitis typically have a history of heavy alcohol use (>100 g per day) for more than 20 years, but heavy drinking may be surreptitious or intermittent, without episodes of obvious intoxication
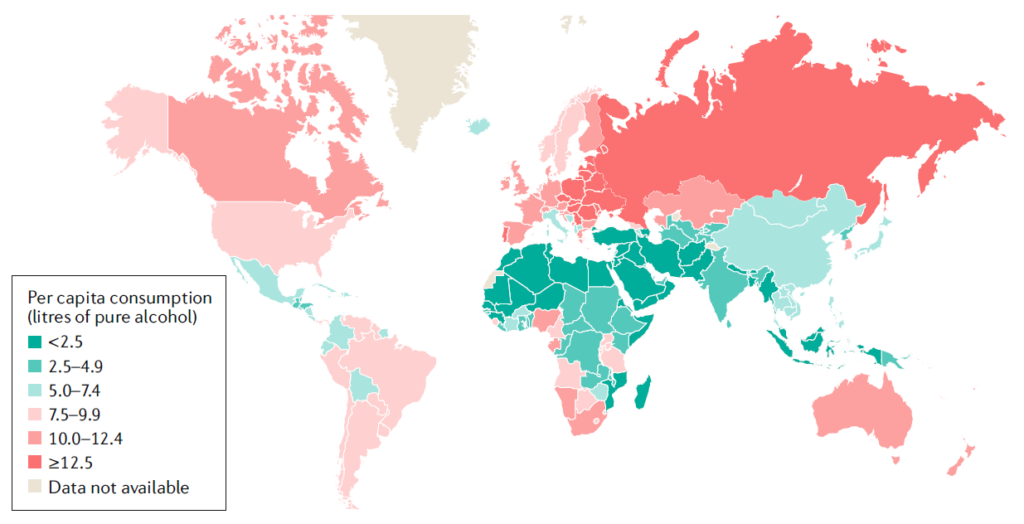
A map showing the total alcohol per capita consumption by región in individuals >15 years of age in 2010. Data from the global WHO report 2014
Clinical manifestations
The characteristic clinical features of alcoholic hepatitis are jaundice, anorexia, fever, and tender hepatomegaly.
Laboratory testing reveals moderately elevated transaminases (typically less than 300 U/L), with an aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio of two or greater. Patients may also present with right upper quadrant/epigastric pain, hepatic encephalopathy, and signs of malnutrition.
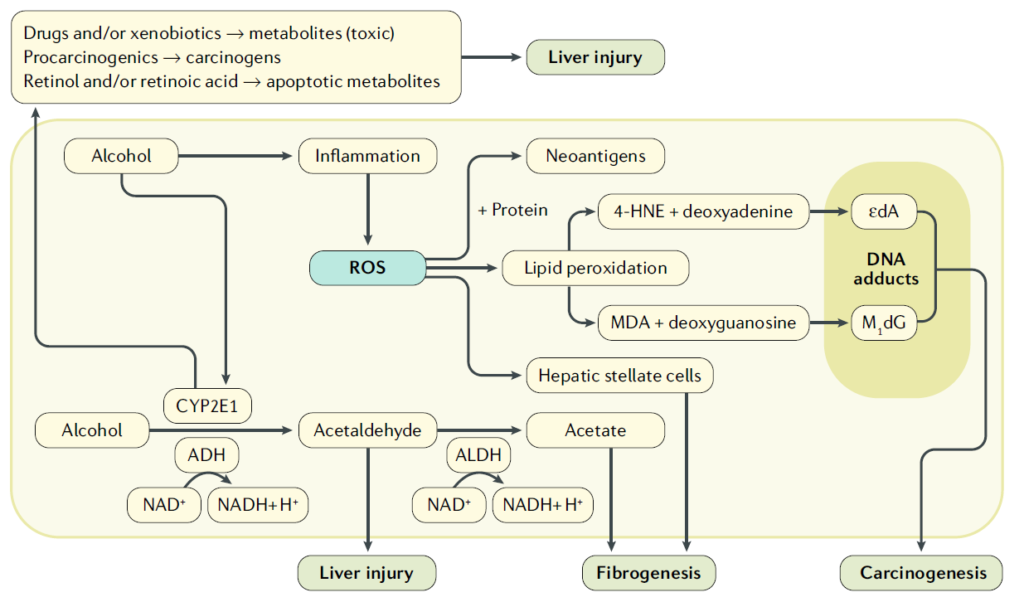
In hepatocytes, alcohol is metabolized to acetaldehyde by alcohol dehydrogenase (ADH), and acetaldehyde is further metabolized to acetate by acetaldehyde dehydrogenase (ALDH). Acetaldehyde is both toxic and carcinogenic. In addition, chronic alcohol consumption results in the induction of cytochrome P450 2E1 (CYP2E1), which also metabolizes alcohol to acetaldehyde. Reactive oxygen species (ROS) are produced as a by- product of CYP2E1 activity. ROS are also generated through inflammation; for example, ROS are generated in alcoholic hepatitis. CYP2E1 also metabolizes some drugs (such as paracetamol or isoniazid) to toxic metabolites, activates procarcinogens to form carcinogens (such as nitrosamines) and degrades retinol and retinoic acid to apoptotic polar intermediates, which can induce hepatic cell death. All these pathways may further contribute to liver injury. In addition, ROS may bind to proteins and generate neoantigens, which are modified host proteins that induce a host immune response. ROS can also lead to lipid peroxidation with the generation of lipid peroxidation products such as 4-hydroxynonenal (4-HNE) or malondialdehyde (MDA). Both compounds can bind to DNA bases with the formation of carcinogenic exocyclic etheno–DNA adducts. ROS can also stimulate hepatic stellate cells, which results in fibrogenesis. Thus, alcohol- generated ROS are responsible for a cascade of negative events that can contribute to the development of alcoholic liver disease. εdA , 1,N6-etheno-2′-deoxyadenosine; M1dG, 3-(2-deoxy-β-d- erythro-pentofuranosyl)pyrimido(1,2-α)purin-10(3H)-one.
Diagnosis
Clinical and laboratory features are often adequate for establishing the diagnosis of alcoholic hepatitis in a patient with a long history of heavy alcohol use, provided the patient does not have risk factors for other causes of acute hepatitis and testing for other common causes of hepatitis is negative.
If the diagnosis is not certain, then liver biopsy can establish alcohol as the likely etiology with greater certainty.
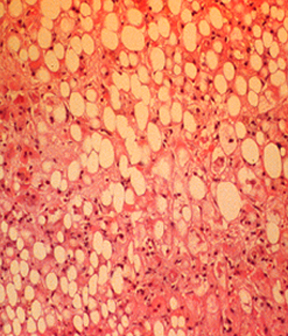
Medium power view of a liver biopsy from a patient with alcoholic hepatitis shows the classic changes of hepatocellular steatosis, neutrophilic infiltration (in contrast to the mononuclear cell infiltration in other forms of chronic hepatitis), and focal hepatocyte necrosis. These changes are indistinguishable from those in nonalcoholic steatohepatitis.
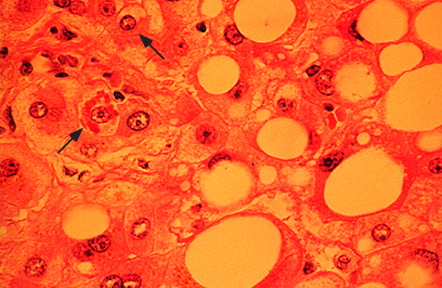
High power view of a liver biopsy in alcoholic hepatitis shows macrovesicular fat and Mallory-Denk bodies (arrows), which are eosinophilic accumulations of intracellular material. Similar changes can occur in nonalcoholic steatohepatitis.
Differential diagnosis
There are numerous causes of acute hepatitis, including viral infection, drug reactions, and ischemia.
The primary features that differentiate alcoholic hepatitis from other causes of acute hepatitis include a history of heavy alcohol use and an AST:ALT ratio of ≥2.
Decompensated cirrhosis can occur concurrently with acute alcoholic hepatitis and confers a higher risk of liver failure compared with those who have alcoholic hepatitis or cirrhosis alone.
Management and prognosis of alcoholic hepatitis.
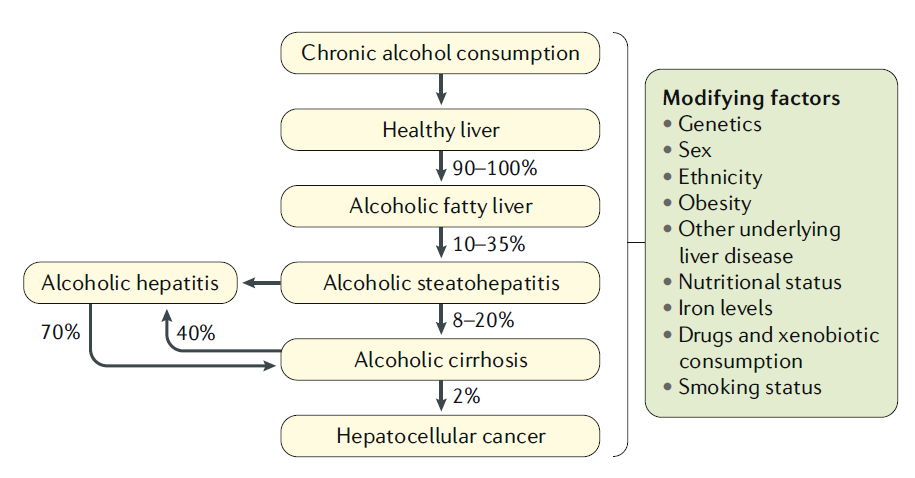
Chronic heavy (>40 g of alcohol per day) alcohol consumption over a sustained period (months or years) will result in 90–100% of individuals developing alcoholic fatty liver. Only 10–35% of individuals with alcoholic fatty liver who continue with chronic heavy alcohol consumption will develop alcoholic steatohepatitis, which is inflammation of the liver characterized by specific histological features. Furthermore, only 8–20% of chronic heavy drinkers will develop alcoholic liver cirrhosis. Of these patients with cirrhosis, ~2% per year develop hepatocelular cancer. Patients with severe alcoholic steatohepatitis may develop the acute clinical entity of alcoholic hepatitis, a disease characterized by jaundice and liver failure. Of the patients who survive alcoholic hepatitis, 70% will develop cirrhosis. By contrast, 40% of patients with alcoholic liver cirrhosis may also develop alcoholic hepatitis (acute- on-chronic disease), with very high mortality rates. The natural course of alcoholic liver disease is modified by various factors (right- hand box). Figure adapted from ref.46, Springer Nature Limited.
Determining the severity
Several prognostic models have been proposed to determine the severity of a patient’s alcoholic hepatitis. The Maddrey discriminant function (DF) and Model for End-stage Liver Disease are the most commonly used to identify patients with severe alcoholic hepatitis (DF ≥32) who are more likely to benefit from pharmacologic therapy. The Lille score has also been well validated.
For patients with severe alcoholic hepatitis, short-term mortality rates are high (approximately 25 to 35 percent at one month), whereas patients with mild to moderate alcoholic hepatitis have low short-term mortality rates (<10 percent at one to three months).
Maddrey’s discriminant function (rccc.eu)
Initial management of all patients
Patients with alcoholic hepatitis require general supportive care, including:
- Alcohol abstinence
- Prevention and treatment of alcohol withdrawal
- Fluid management
- Nutritional support
- Infection surveillance
- Prophylaxis against gastric mucosal bleeding
In addition, we suggest that nonselective beta blockers are discontinued in patients with severe alcoholic hepatitis.
Patients with mild to moderate alcoholic hepatitis
The mainstay of treatment for patients with mild to moderate alcoholic hepatitis is abstinence from alcohol and supportive care.
In patients with mild to moderate alcoholic hepatitis (DF <32), we recommend against pharmacologic therapy with prednisolone.
Prednisolone does not appear to be beneficial in such patients. In addition, we suggest against pharmacologic therapy with pentoxifylline.
Pentoxifylline has not been studied in this population, and its efficacy in patients with more severe alcoholic hepatitis has not been established.
Patients with severe alcoholic hepatitis (DF ≥32)
In patients with severe alcoholic hepatitis (DF ≥32), we suggest treatment with glucocorticoids (40 mg per day) along with supportive care. If there are no signs of improvement (ie, decreasing bilirubin or DF) after one week of therapy, we stop glucocorticoid treatment; otherwise we treat for 28 days.
However, not all authorities agree with this recommendation, instead favoring pentoxifylline in certain populations.
This includes patients with contraindications to glucocorticoids as well as those whose social circumstances place them at risk for poor continuity of care.
As an example, patients who are homeless and given a prescription for glucocorticoids may not receive proper follow-up to ensure that the glucocorticoids are discontinued after 28 days.
By contrast, the safety profile of pentoxifylline (400 mg three times per day [or once daily in patients with a creatinine clearance <30 mL/minute]) is relatively favorable in such settings.
A bilirubin less than 5 mg/dL may be an appropriate time to stop pentoxifylline.
Pentoxifylline should be stopped earlier in patients who develop dyspepsia that limits oral intake.
 Cargando…
Cargando…